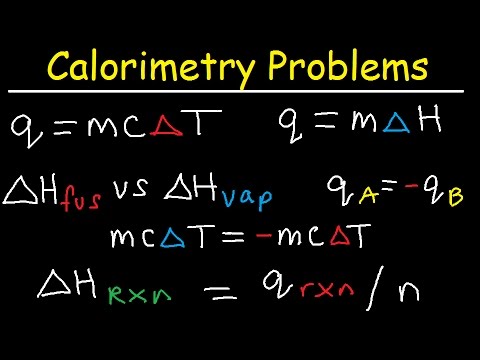
 This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. It shows you how to calculate the quantity of heat transferred using specific heat capacity during a temperature change process and using the enthalpy of fusion and heat of vaporization during a phase change process. This video contains plenty of examples and practice problems.
This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. It shows you how to calculate the quantity of heat transferred using specific heat capacity during a temperature change process and using the enthalpy of fusion and heat of vaporization during a phase change process. This video contains plenty of examples and practice problems.
Here is a list of topics:
1. Calorimetry
2. Thermochemistry Practice Problems
3. How to calculate the amount of energy required to heat water
4. Specific Heat Capacity of Water, Ice, and Steam
5. Heat Transfer Problems - Finding the Specific Heat Capacity of the Unknown Metal
6. How to determine the amount of heat needed to melt ice
7. Heat of Fusion vs Heat of Vaporization
8. How to calculate the final temperature of the mixture between iron metal and water.
9. Heating curve of water
10. How to calculate the enthalpy of a dissolution reaction
Calorimetry Problems, Thermochemistry Practice, Specific Heat Capacity, Enthalpy Fusion, Chemistry watershed | |
| 1,897 Likes | 1,897 Dislikes |
| 212,816 views views | 1.16M followers |
| Education | Upload TimePublished on 18 Dec 2016 |
No comments:
Post a Comment